A more academic version of this article is available as a PDF file for you to download and keep. Click to access.
Fortunately, once the doublethink has been acknowledged, it should be possible to build a second generation of wind turbines that will allow all nations to achieve carbon neutrality several years before their agreed targets. Second generation wind energy will be cheap and reliable, so living standards will improve too.
----------------------------------------
The new thinking in a nutshell
The second generation wind turbines will be ‘canned’, with a fan being used to generate an artificial wind inside a closed loop of conduit.
There is a Venturi constriction in the loop, where the wind speed increases. Consequently, a correctly design turbo-generator inside the Venturi throat will generate more electricity than is required to power the fan The energy balance is maintained by the circulating air cooling below ambient. This draws in heat from the surrounding environment so that the system as a whole acts as a heat engine that runs on atmospheric heat.
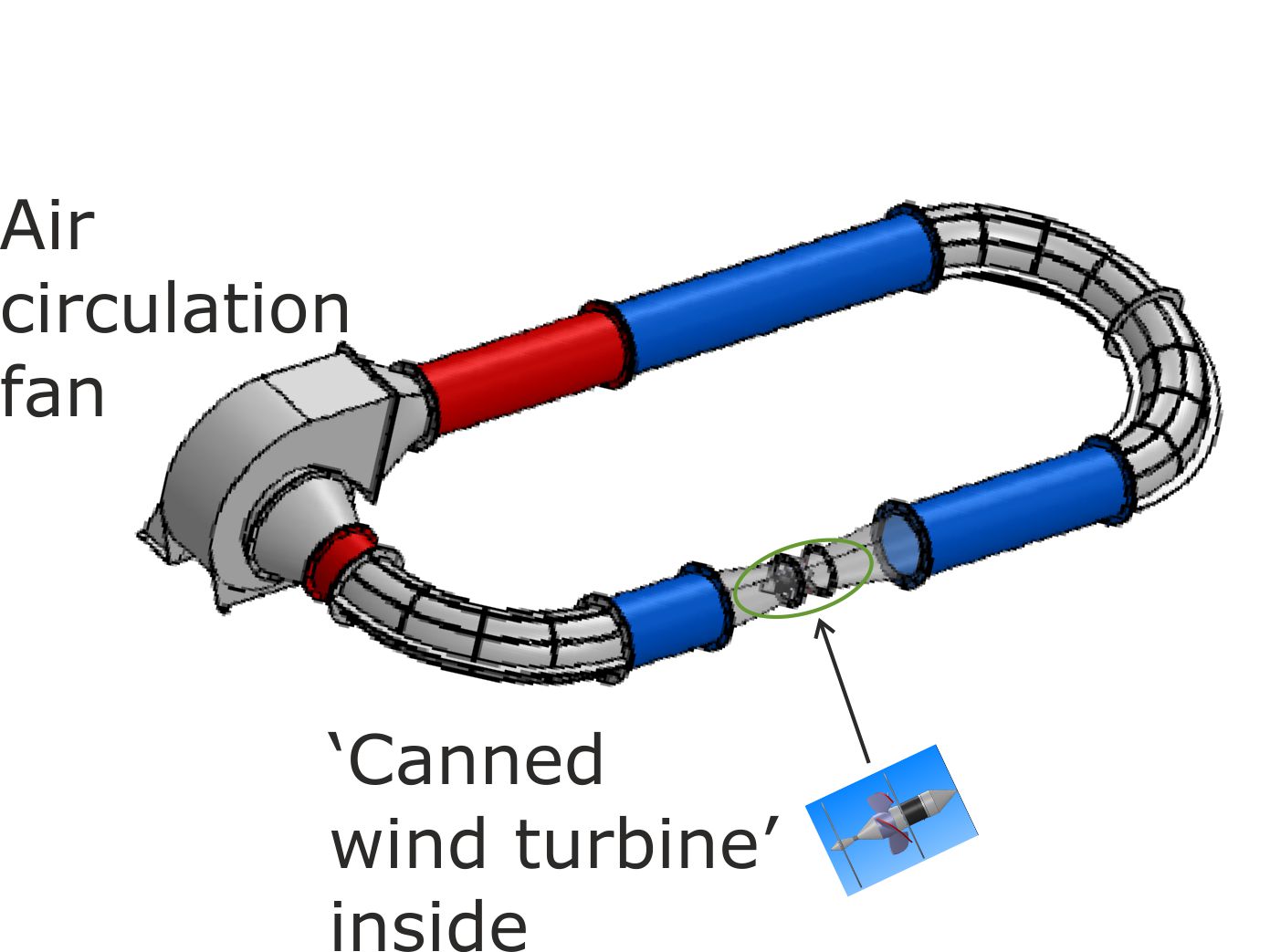
If you are unfamiliar with engineering thermodynamics, then this design will probably seem quite plausible. But, if you have been taught engineering thermodynamics, then you will have been conditioned into believing that it is thermodynamically impossible to build a heat engine that runs on atmospheric heat (thermal energy).
There are two reasons why the textbook engineering teaching has gone wrong. First, the term ‘heat’ is used ambiguously in science and second; Carnot’s 1824 concept of a heat engine was never updated to take into account the discovery of internal energy around 1850.
The aim of this article is to explain why the standard engineering teaching on heat engines is wrong.
----------------------------------------
The second generation wind turbines (Latent Power Turbines) referred to are open source technology, meaning that anyone reading this article is free to develop them as they wish.
Heat engines convert thermal energy into mechanical energy. Manufactured heat engines that burn fossil fuels power most of our transport systems and generate most of our electricity. Natural heat engines include all of the convection currents found in the Earth’s atmosphere and oceans. Scientists accept the existing explanations of how natural and manufactured heat engine work, even though these explanations are contradictory.
According to the accepted science:
(i) It is impossible to build circular heat engines that recycle their rejected thermal energy because heat cannot travel from cold to warm.
(ii) The convection currents found in nature are proof of the existence of circular heat engine systems.
So, according to the doublethink science of heat engines, we have proof of the existence of circular heat engine systems, even though it is thermodynamically impossible for them to exist.
Heat sinks are the scientific concept that has caused our doublethink problems.
Engineers are taught that all heat engines must have heat sinks to dissipate their rejected thermal energy. But if all the natural heat engines that drive our atmosphere were as wasteful in their use of heat sinks as manufactured heat engines, the atmosphere would stagnate and there would be no weather changes.
Heat engines are both the heroes and the villains in the climate change story.
The heroes
These are the natural heat engines that power our weather systems and make life on land possible by shifting water from the sea to the land. Their original source of thermal energy is radiant heat from the sun. First the sun warms the land and the oceans, which then warm up the atmosphere. These heat engines are more efficient than their manufactured equivalents, even though they run far cooler, usually having temperatures well below 50oC.
Ina nutshell, the reason for this higher efficiency is that they have less heat sinks.
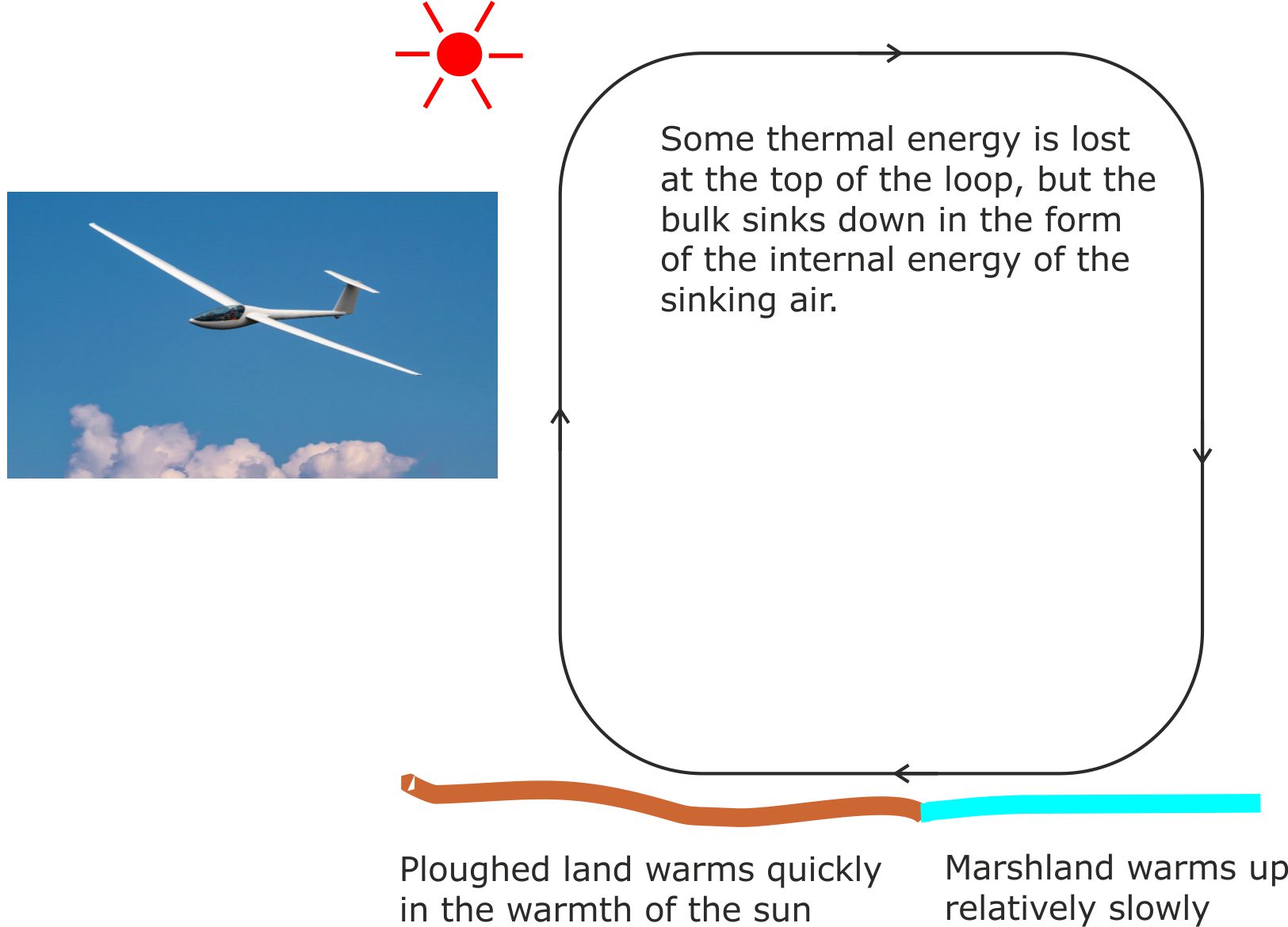
Fig 1. This glider is being lifted up against gravity by a rising current of warm air.
The air involved is part of a convection loop caused by slight variations in the rate at which different surfaces warm the air above them. For example, ploughed agricultural land warms overlying air faster than an adjacent area of marshland.
At the top of the convection current loop, some thermal energy is lost into a heat sink, but the bulk of the thermal energy remains stored in the sinking air.
This natural heat engine system is very efficient because the bulk of the thermal energy stored in the air just keeps on being recycled round and round the convection loop.
The villains
These are the heat engines that burn fossil fuels to power our transport systems and generate electricity. These villains pump carbon dioxide (CO2) into the atmosphere and cause global warming. They also make a small contribution to global warming more directly by dumping low grade heat into the atmosphere.
All of these manufactured heat engines run hot. Typically they have thermal energy input temperatures at least a hundred degrees warmer than the atmosphere anywhere on our planet. Even their output temperatures are higher than that of their environment. This temperature difference allows them to dump their rejected thermal energy into the environment in the form of heat.
The success of hot running heat engines in keeping modern society running adds common sense support to the belief that the engineering scientists have got it right and that all heat engines require heat sinks.
But the heat engines that combine to create hurricanes and other wind phenomenon also require a second type of thermal energy sink in order to function.

Fig 2. The fundamental reason why hurricanes are so efficient is that they employ two different types of thermal energy sinks. Some are heat sinks which dissipate thermal energy, but many are non-dissipating sinks, which allow thermal energy to be recycled. It is these non-dissipating sinks which allow heat engines to work together in chains to create circular convection current loops.
In contrast, all of the mass produced manufactured heat engines are wasteful linear systems that only employ dissipating heat sinks.
There is a terrible irony here because since 1859 we have had the scientific evidence that adding carbon dioxide to the atmosphere will make natures circular heat engines too efficient, causing the atmosphere to warm up and weather conditions to become more extreme. [John Tyndall: the forgotten co-founder of climate science ...]
Meanwhile, the engineering scientists who we rely on to invent us out of the climate crisis are handicapped by believing that circular heat engine systems are thermodynamically impossible.
This is yet another example of doublethink science because scientists are aware that increasing the input temperature to a heat engine can also increase its efficiency. But the engineering and meteorological scientists come to startlingly different conclusions.
For example, engineering scientists using the Carnot equation (details later on this page) calculate that increasing the temperature of the steam input to a power station turbine by 4oC will (at best) improve heat engine efficiency by a modest 0.2%. However, the meteorological scientists warn us that if the Earth’s atmospheric heat engine warms up by 4oC, the consequences will be apocalyptic.
[Impacts of a 4°C global warming: 1. A 4°C world]
Both of these predicted consequences of a 4oC increase in temperature are realistic, with the difference being that a power station turbine is a linear system, whereas the Earth’s assembly of atmospheric heat engines is a circular system.
Unfortunately doublethink kicks in, so while accepting these dire predictions, the power station designers are also conditioned into believing that such efficient circular heat engines are thermodynamically impossible for them to build.
Heat sinks and their limitations
Heat engines cannot convert thermal energy into mechanical energy with 100% efficiency.
During their operation, they always produce some low grade (relatively cool) thermal energy that they have to dispose of. According to the engineering textbooks, this must be disposed of using heat sinks. Nature’s heat engine systems also require heat sinks, but to create convection current loops, they also need some non-dissipating sinks.

Fig 3. If you pour boiling hot water down this kitchen sink, it soon becomes mixed in with all the other water in your local drains. Consequently, the increase in temperature of the drain water is low. The drains are acting as a heat sink, reducing the temperature of the thermal energy you poured away with the water.
At a molecular level, the fast moving molecules in the boiling water mingle with the slower moving molecules in the cool drain water, transferring some of their kinetic energy to them. So pouring boiling water down the sink does not destroy thermal energy, it just results in the thermal energy being shared between more molecules. Consequently, the thermal energy is less accessible. For example, you can brew a decent cup of tea or wash greasy plates using the original boiling water, but once the thermal energy has been reduced to drains temperature, neither task is possible.
Hurricanes and other atmospheric convection currents are essentially millions of tiny heat engines linked together in long closed chains. If all the heat engines in hurricanes had to rely solely on energy dissipating heat sinks, then hurricanes could never become more powerful than gentle breezes.
So, circular heat engines must involve some other kind of sink that prevents the thermal energy from dissipating.
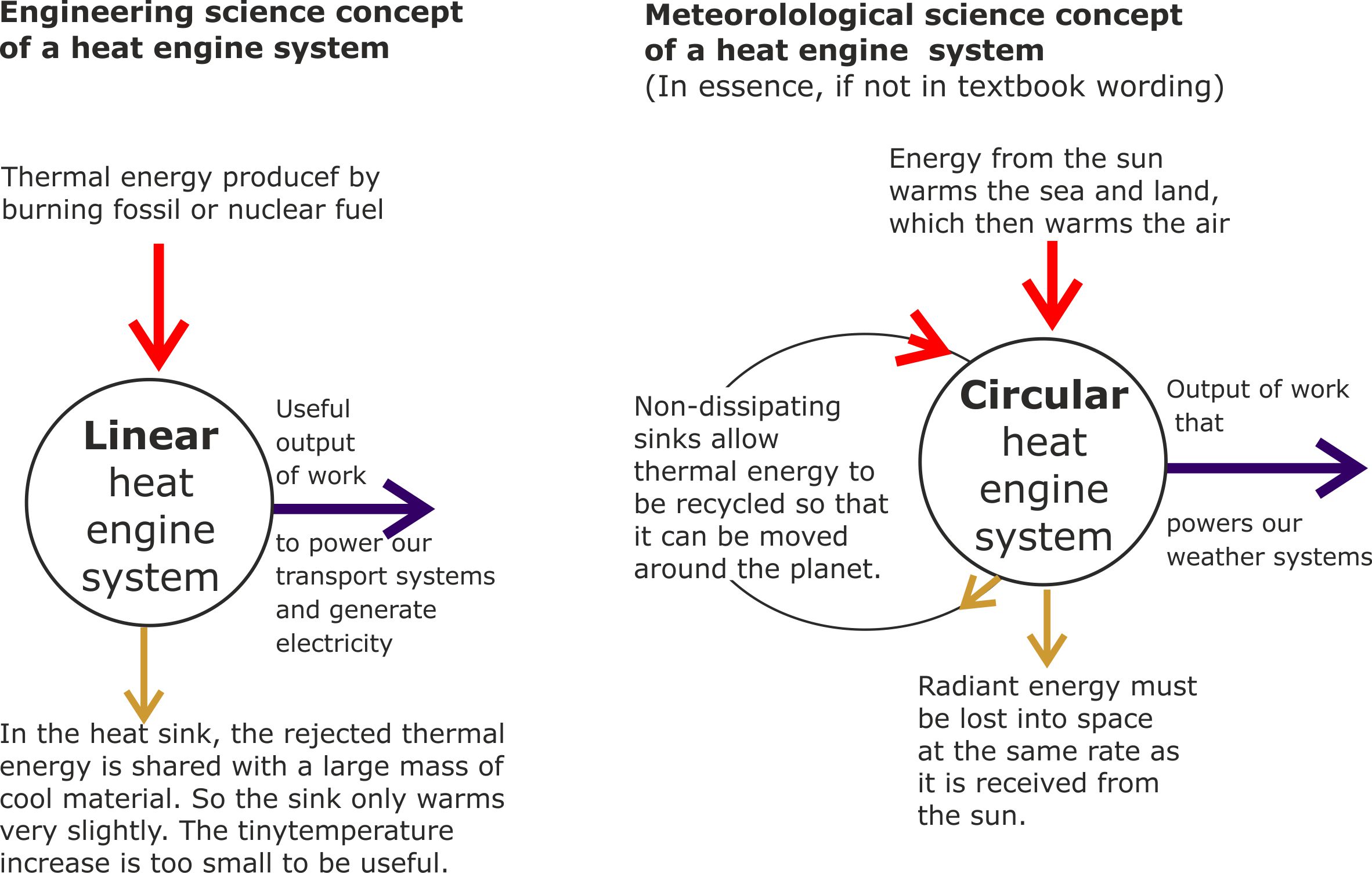
Fig 4. For the last 150 years or so, meteorologists have been well aware that the circular heat engines of nature are consistent with the laws of thermodynamics. But for a similar period of time, engineering students have been taught that it is impossible to construct circular heat engines because they would defy the laws of thermodynamics.

Fig 5. Engineers designing linear heat engines always have to allow for the disposal of low grade thermal energy. Most commonly, this energy is dumped into the atmosphere.
In contrast, meteorologists have no thermal energy dissipation problems to deal with because the Earth only has one atmosphere. So, all the energy rejected by natural heat engines has to go back into the same atmosphere that it came from.
On the other hand, the atmosphere still needs outer space to act as a radiant heat sink to ensure that the Earth is not overheated by the sun.
However, the crucial difference between engineers and meteorologists is that engineers are still locked into Carnot’s 1824 heat engine concept, which only allows for heat travelling from hot to cold. In contrast, meteorologists recognise that the thermal energy stored atmospheric air can move ‘the wrong way’, from cold to warm because pressure differences and not temperature differences move air around.
Internal energy and heat
Before we discuss the alternative to heat sinks, we need to distinguish between two different forms of thermal energy, internal energy and heat.
Originally, in Old English ‘heat’ meant warmth, with this still being the generally understood meaning of the word today. However, in thermodynamics, it has a distinctly different meaning that has contributed generously to the founding of doublethink science.
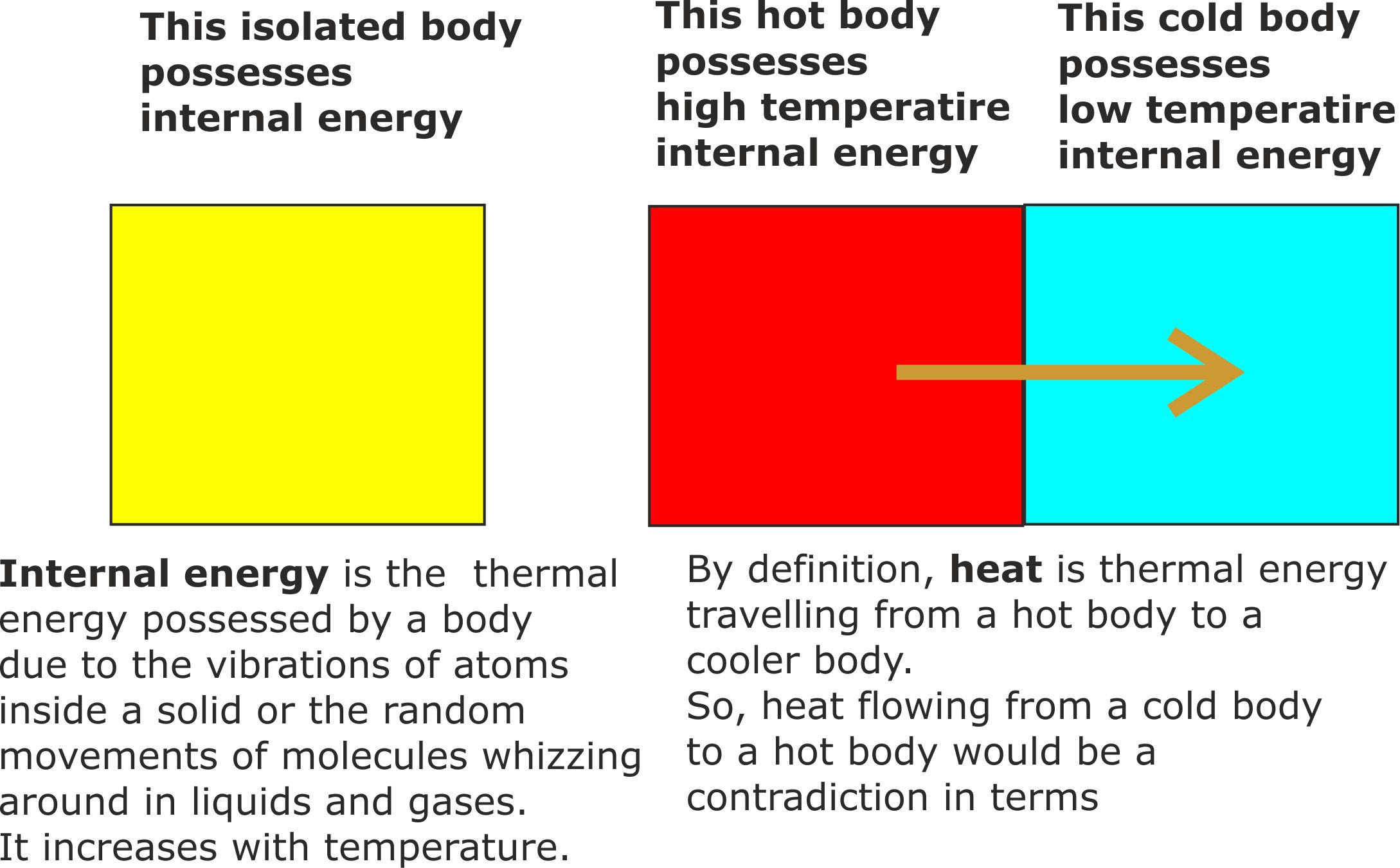
Figure 6. Internal energy is the energy stored inside a body thanks to the vibrating atoms in a solid, or the molecules whizzing round randomly inside a liquid or gas.
A body at a high temperature has more internal energy than when it is cold.
So the temperature of a body is a measure of the average energy of the vibrating atoms or randomly moving molecules.
Heat is energy in transfer from a warm body to a cooler body. Bodies do not store heat, they store internal energy.
This common error highlights another example of doublethink.
To avoid being wordy, even the experts tend to use the word heat inaccurately when strictly they are referring to internal energy.
For example, an expert might say,
“Ocean currents move heat from the equator to the poles.”
When strictly speaking, what they mean is,
“The water in ocean currents acts as a vector, moving thermal energy from the equator to the poles”.
Such scientific accuracy would irritate the general public and put many people off science. So, for the sake of getting the bigger messages out, especially about global warming, we tolerate this misuse of language.
The problem is that after several generations of scientists using this convenient verbal shorthand, it has become doublethink; with the vector transfer of thermal energy taking on the attributes of heat. This has serious entropy implications that will be discussed later.
Work and what Joule learned on his honeymoon
Another important term we need is work, with energy being defined as ‘the ability to do work.’ [Work = Force x Displacement]
A moving fluid has kinetic energy that can be calculated from the mass and speed of the fluid involved. At a molecular level, this kinetic energy is due to the molecules drifting in the direction of flow. This is in addition to the fluid having internal energy due to the random movement of the molecules. If the fluid is brought to a halt without doing work, then the kinetic energy due to the drift movement of the molecules is converted into internal energy and the fluid warms up.
The eminent scientist J P. Joule famously demonstrated this on his honeymoon, but that’s another story!
When a moving fluid does work, there is a backward force on the fluid, tending to slow it down. But it has to keep moving forward inside a heat engine, otherwise, the engine would become clogged up.
So the reverse of Joule’s discovery happens: the fluid regains kinetic energy due to drift movement at the cost of losing kinetic energy in the form of internal energy.
At a macroscopic level, we detect this change in the roles of kinetic energy as a cooling of the fluid.
The laws of thermodynamics, backed up by observations tell us that that it is impossible to convert all of the random movement of the molecules into the ordered movement of flow. Hence, the fluid must always emerge from the engine having some internal energy.
Doublethink again: not all heat engines are heat engines
The steam engines and Stirling cycle engine that were employed by industry in the first half of the nineteenth century appeared to meet the criteria of being heat engines that required heat sinks.
(i) Steam engines

Figure 7. Early steam engines discharged their rejected thermal energy into the atmosphere in the form of steam. This condensed in the cooler atmosphere to produce clouds of tiny water droplets.
(ii) Stirling cycle engines (patented in 1816) rely on the alternate warming and cooling of the same mass of working fluid.
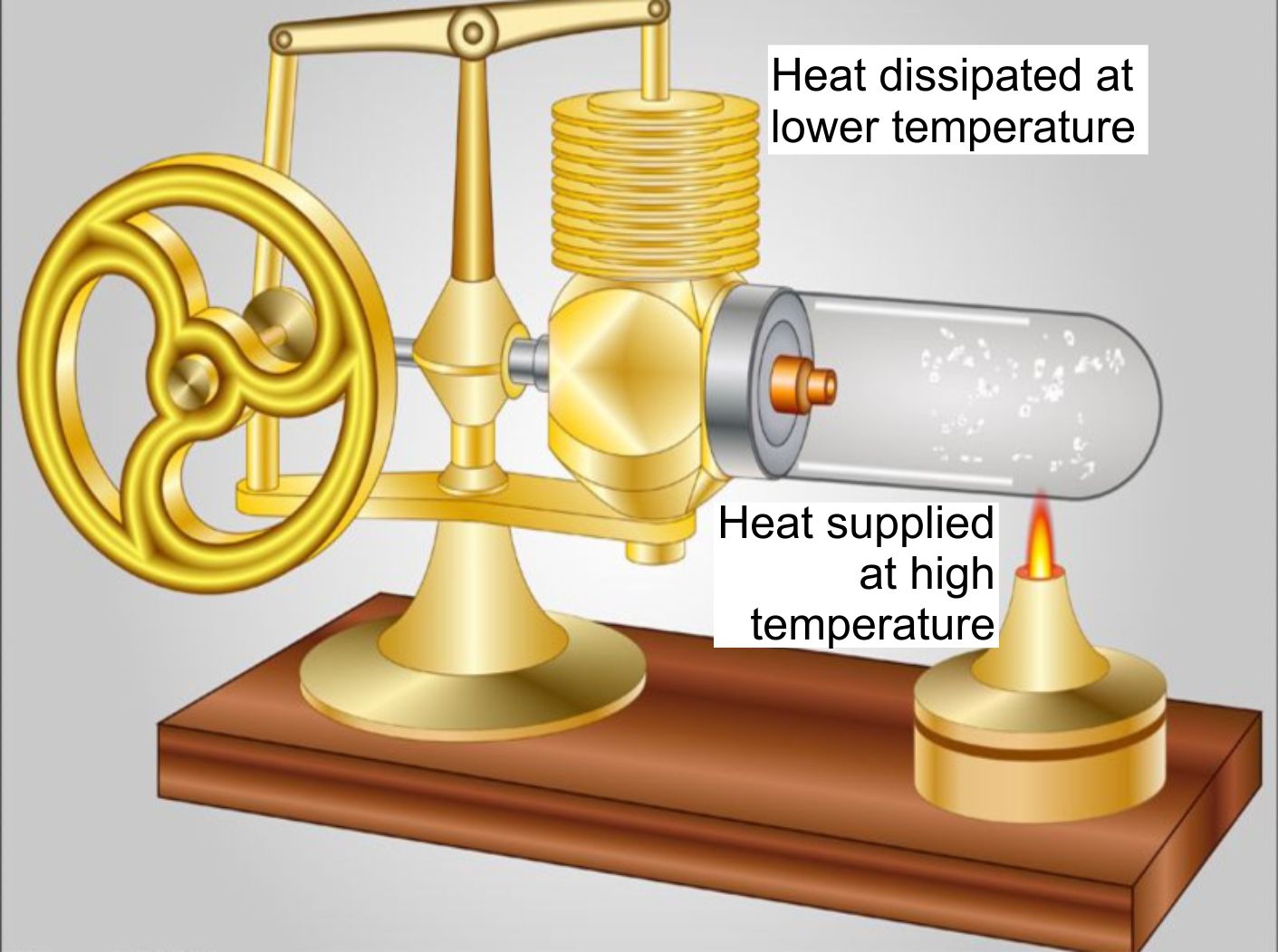
Figure 8. Stirling engines run on heat supplied by a hot external source and dump their rejected thermal energy into a cooler environment as heat.
Stirling cycle and steam engines are both accurately described as ‘heat engines’ because their working fluids are warmed by external combustion sources at a higher temperature.
However, when we move into the second half of the nineteenth century and the invention of internal combustion engines; the generic title of ‘heat engines’ becomes misleading. Heat is replaced by chemical energy, with a fuel plus air mixture burning inside the engine to produce a hot, high pressure working fluid.
When pressed, engineering scientists usually acknowledged that the generic term ‘heat engine‘ is inaccurate. But their tacit acceptance of a misleading generic name is dangerous because (we will argue), it leads on to doublethink, with natural and manufactured ‘heat engines’ supposedly obeying the same laws of thermodynamics, yet having different thermodynamic properties.
In the last decade of the nineteenth century, cool running ‘heat engines’ made an appearance in the form of commercially available pneumatic (compressed air) tools. These convert internal energy stored in compressed air into useful work. As a consequence of doing work, the compressed air loses pressure and its temperature falls.
Dissipating and non-dissipating sinks
The following pair of diagrams introduce the concept of a non-dissipating internal energy sink.
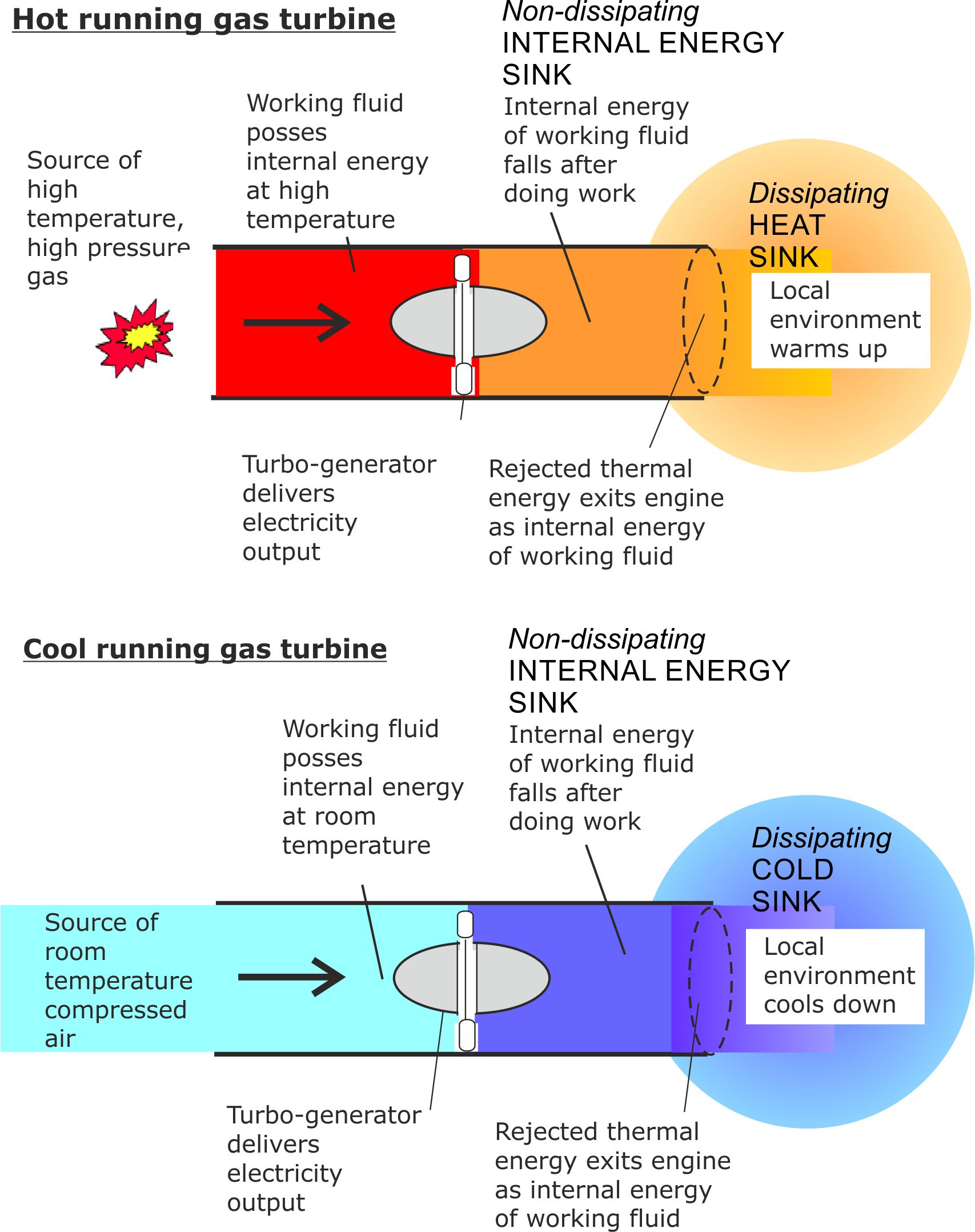
Figure 9. There are no temperature limitations on the exiting fluid, but it must exit at a higher pressure than the surrounding environment.
Here are some further examples to show that some heat engines require dissipating heat sinks, and others, non-dissipating internal energy sinks.

Figure 10. This is a selection of heat engines that have different sink requirements.
Steam turbines eject low pressure steam that condenses out after giving up its latent heat to cooling water inside a heat exchanger. The cooling water then gives up thermal energy to the atmosphere inside cooling towers. This system passes the ‘heat sink test’ because the efficiency of the steam turbine varies with the temperature of the secondary cooling water, which varies with the temperature of the final heat sink, i.e., the surrounding atmosphere.
Rockets in the vacuum of space: Internal energy is ejected in the form of the exhaust gases.
A water cooled car engine has three sinks:
(i) Heat is dumped directly into the cooler atmosphere via the hot engine block; no internal energy sink is required.
(ii) Heat passes indirectly into the cooler atmosphere via the radiator. In this case, the cooling water acts as a vector, carrying the thermal energy from the block to the radiator.
(iii) The local atmosphere acts as an internal energy sink for the combustion chamber exhaust gases.
The external combustion steam engine (Again)
With the benefit of hindsight it can be seen that steam engines require internal energy sinks because they discharge exhausted steam that has internal energy into the environment, not heat. Such an engine would not be able to function in a very high pressure environment, for example, on the surface of Venus, because the exhausted steam would be at a lower pressure than the environment.
The caloric theory of heat, entropy and boundary conditions
It was only after the concepts of atoms and molecules had become widely accepted that thermodynamics took over from caloric theory as the science of heat
Caloric was the name given to a hypothecated fluid that could store warmth but was invisible and weightless. The warmer a body, the more caloric it supposedly contained. If a warm body was placed in close contact with a cooler one, caloric supposedly flowed from warm to cold until the bodies were in equilibrium. According to caloric theory, there would be little difference between a heat sink and an internal energy sink.
It is speculated by the author, that belief in the absolute supremacy of heat sinks for all heat engines is a legacy from this caloric era.
However, once we enter the age of thermodynamics, the difference between the two types of sinks becomes critical because they have different entropy properties.
The following diagram explains the difference.
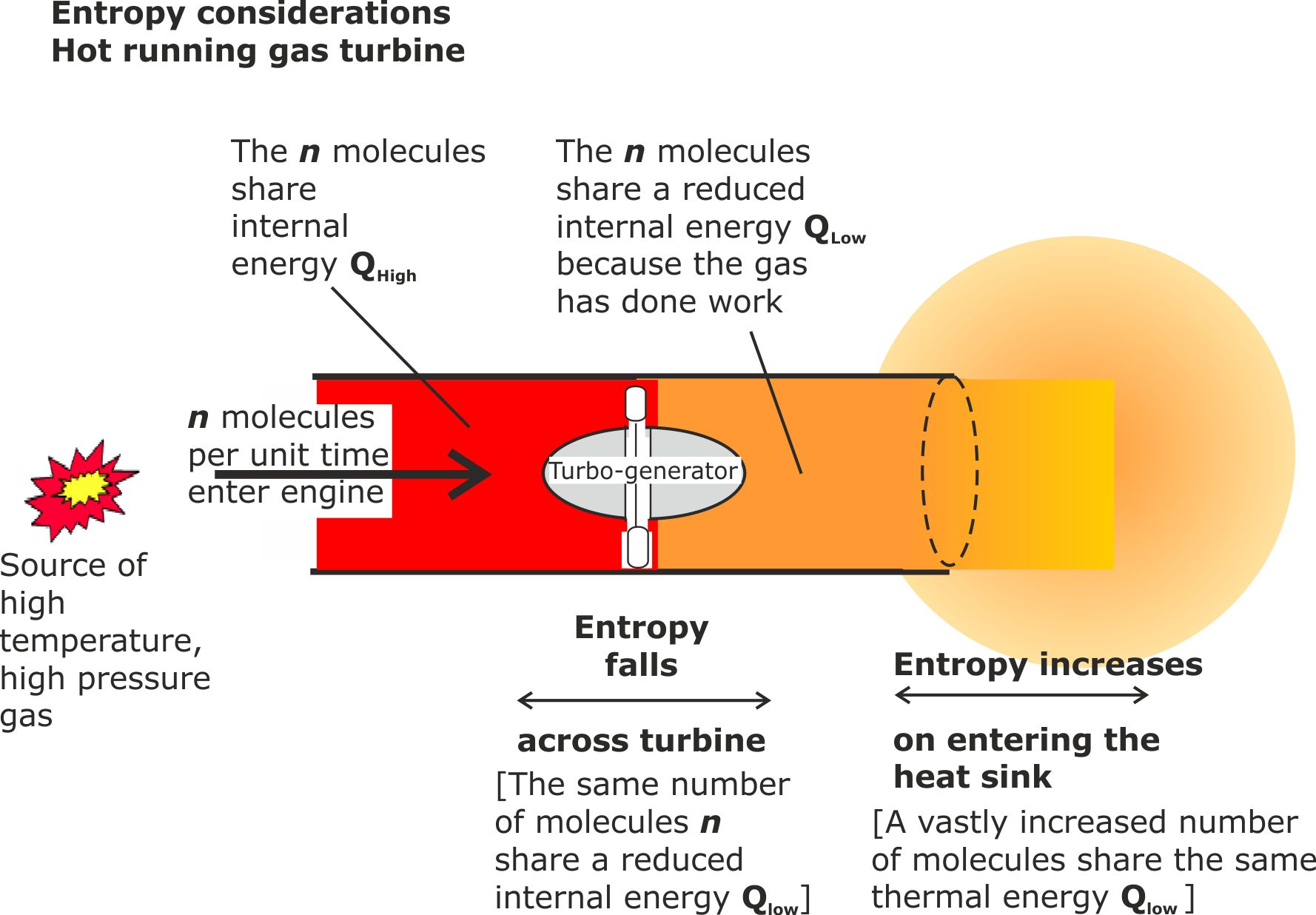
Figure 11. Entropy falls for thermal energy entering an internal energy sink, but rises when it enters a heat sink.
In addition, the two types of sink also have different boundary conditions.
(i) Thermal energy can only enter a heat sink at a lower temperature.
(ii) The internal energy stored in a working fluid can only enter an internal energy sink at a lower pressure.
Diagrams summarising the basic properties of heat engines
(i) Existing learning
This is a standard heat engine diagram, typical of pre-university physics textbooks.
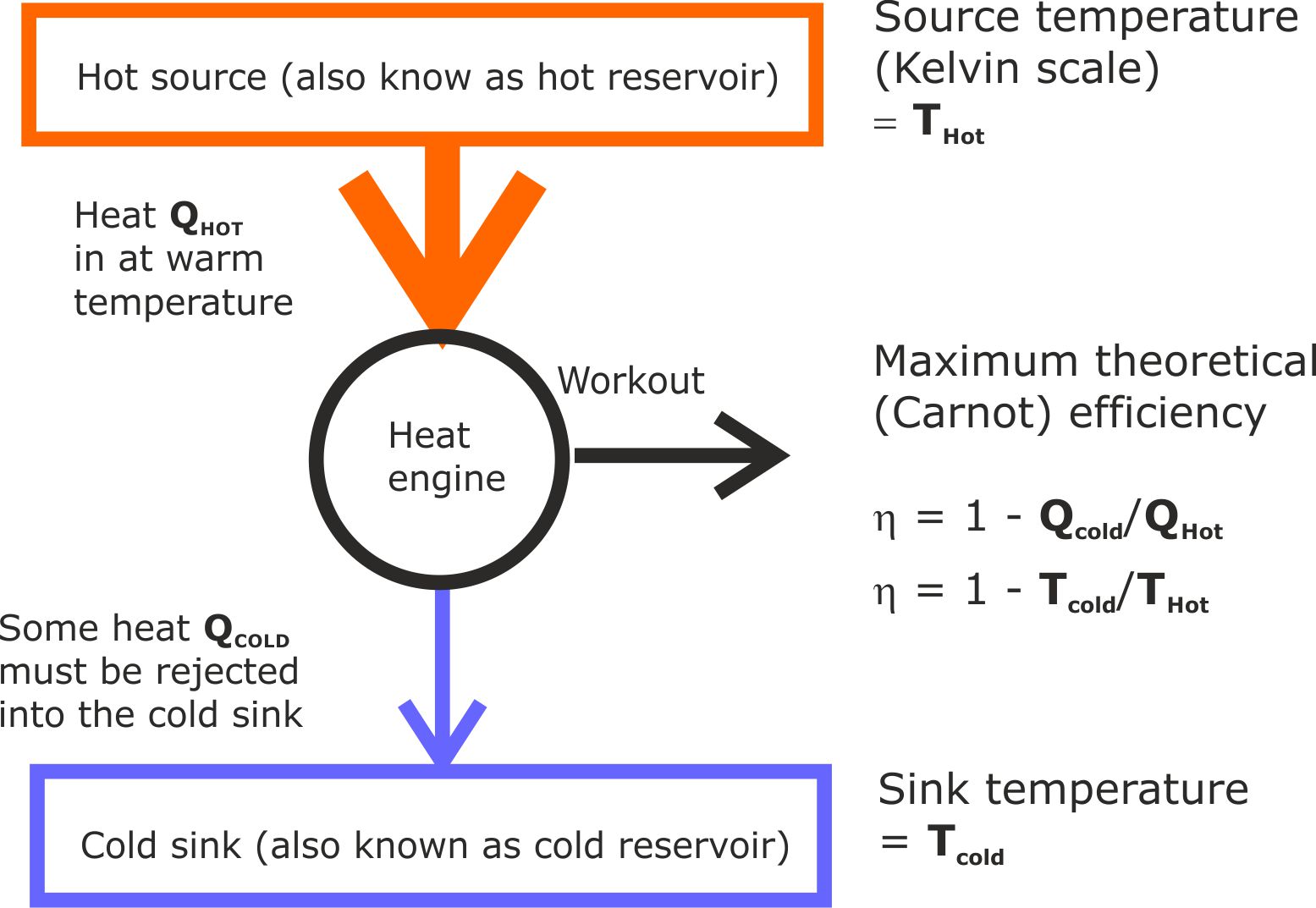
Figure 12. This diagram summarises the key concepts students are expected to grasp about heat engines.
(ii) Proposed new learning
The single diagram has to be replaced by two diagrams if the existence of internal energy sinks is accepted.

Figure 13. These diagrams are new, but the scientific information they communicate has existed under our noses since the nineteenth century. It is only the mental blockage caused by scientific doublethink that has prevented us from seeing them.
They provide a tool for teaching future generations of scientists that there are two different types of thermal energy sink, only one of which allows recycling. Hopefully this will lead to new types of circular heat engines being invented in the future.
The different properties of the two types of sink only become importance when discussing circular heat engine systems.
This means that all of the existing textbook information about manufactured heat engines is perfectly adequate, but limits the trainee engineer’s creativity to developing new linear heat engines.
When designing any future circular heat engine systems, two limitations will need to be born in mind.
(i) Circular internal combustion engines are ruled out because their exhaust is chemically different to the pre-ignition fuel and air mixture.
(ii) Circular steam turbines, where the steam is allowed to expand as it moves through the turbine, in order to avoid condensation and blade pitting are also problematic because work will have to be done recompressing the steam.
Natures circular heat engine systems
The following diagram shows a night time coastal breeze system.
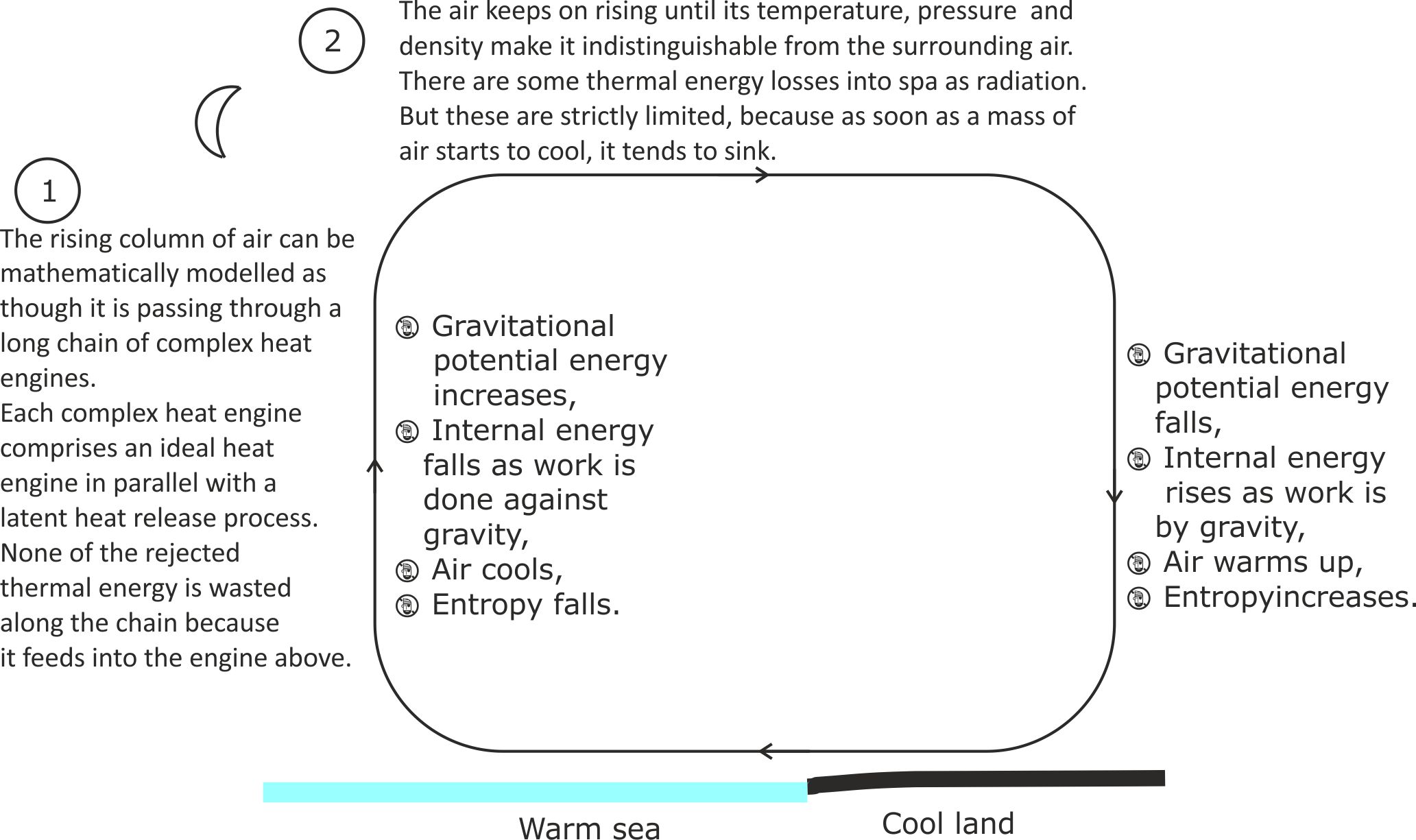
Figure 14. The rising column of air can be considered as a vertical chain of heat engines, with the first engine at the bottom of the column feeding rejected thermal energy into the engine above it and so on. Thus, all the engines in the chain have internal energy sinks.
However, the final heat engine at the top of the rising air column has both heat and internal energy sinks. A heat sink must be operating, otherwise the air at the top of the column would eventually warm to the same temperature as at sea level and all circulatory air movements would cease. On the other hand, if all of the thermal energy at the top of the column was dumped to a higher level in the atmosphere or radiated into space, the air would cool until it liquefied and then fell to absolute zero.
In general, ground level temperature differences of just a few degrees are required to create the thermals that keep glider aircraft aloft. So air that has sunk to ground level will only have lost a few percent of its internal energy, compared with air that is just about to rise. This tells us that the thermal energy loss into the heat sink at the top of a convection current is low, compared with the thermal energy recycled via the internal energy sink. It is also far lower than for manufactured heat engines, which are typically less than 50%.
As the temperature difference between the land and the sea falls, the more sluggish the convection current becomes. But this also means that the temperature difference between the air that has just sunk to sea level and the air that is just about to rise has also fallen. This implies that less thermal energy has been lost at the top of the convection current.
Consequently, the thermal efficiency of this natural heat engine system tends towards 100% as its performance becomes more sluggish.
[Estimating the thermal efficiency of a convection current loop by comparing the temperature of the air on arrival and departure from ground level is only strictly accurate for dry air, where there are no latent heat complications. Nevertheless, it provides an illuminating comparison with linear heat engine systems where engineers strive to maximise the temperature difference between input and output in order to maximise thermal efficiency.]
Another key feature to note about convection currents in general is that the sinking fluid passes through a second chain of heat engines, but working in reverse. That is, they convert mechanical energy in the form of potential energy into internal energy.
We can tap into convection currents by adding First generation wind turbines aka, those we employ today.
These are essentially heat engines that run on thermal energy extracted from atmospheric air. But they are only able to do so indirectly, after nature has employed convection currents to convert thermal energy stored in the air into the kinetic energy of winds.
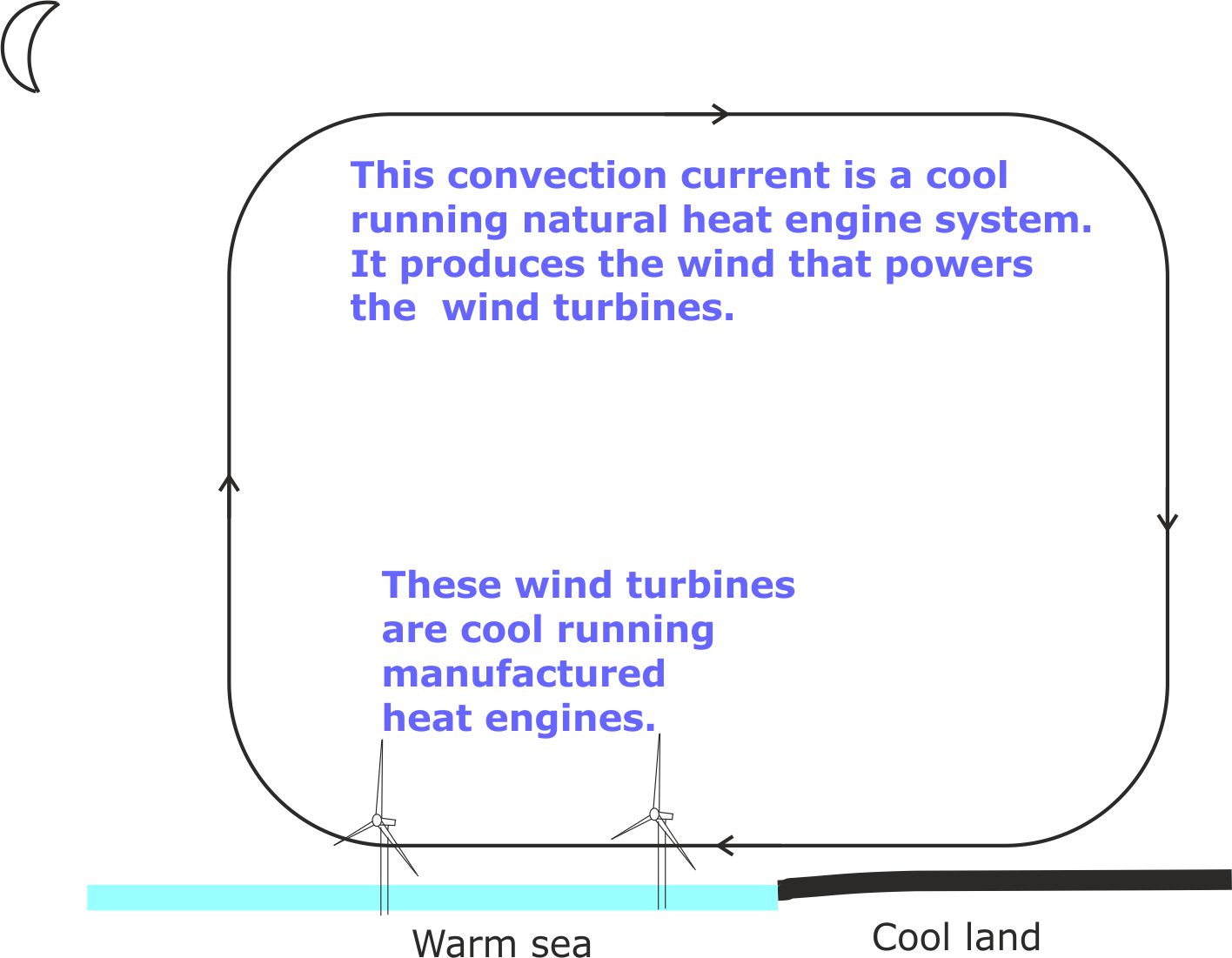
Figure 15. Wind turbines produce low carbon energy, but their output depends on the whims of the wind.
According to current engineering science thanking, the bulk of our future renewable energy needs will be met using large outdoor wind turbines and solar panels.
But, inspired by nature’s convection currents, we can do far better than this!
To bring wind energy under our control, we will need to employ ‘canned wind turbines’ operating in a controlled environment where the ‘wind’ strength. Can be regulated.
Some design tools for building second generation wind turbines
(i) To eliminate the need for tall columns of air, potential energy changes will be replaced by changes in kinetic energy.
The ‘canned wind turbine’ will be added at the point of maximum kinetic energy. But because it will not be operating in the open air, gas turbine style turbine blades will be more effective than conventional wind turbine shaped blades.
[To keep the equations simple, the air will be treated as an incompressible fluid. This is true, to a good first approximation.]
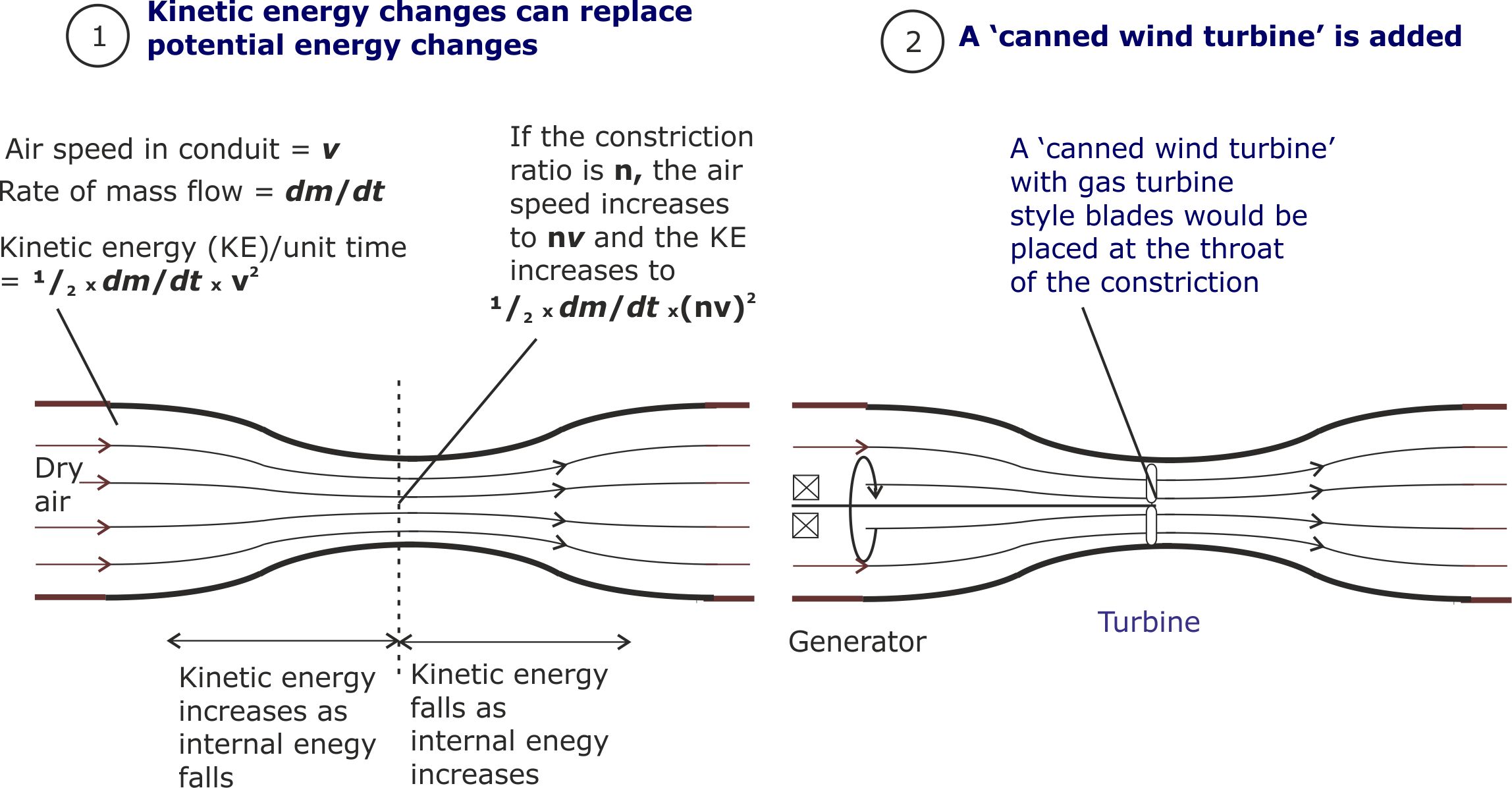
Figure 16. Kinetic energy increases with the square of air speed. So replacing potential energy with kinetic energy makes the second generation wind turbine systems more compact.
(ii) To create a substitute for the wind, a fan will need to be added. By placing the fan in the widest part of the conduit, where the ‘wind’ speed is lowest, the fan will not need to work as hard as if it was placed next to the turbine in the conduit throat.
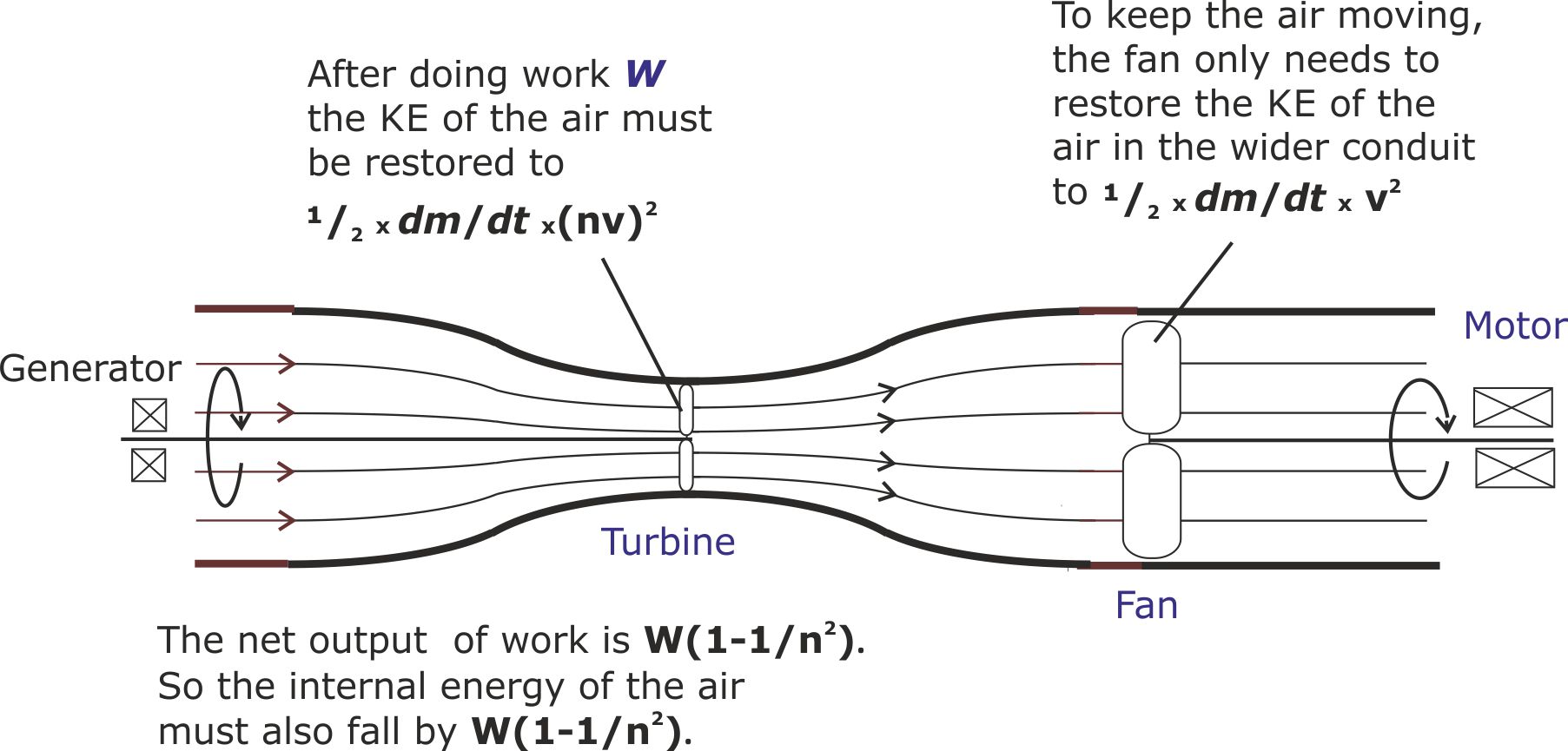
Figure 17. The fan restores the air to full speed throughout the conduit, but the amount of work done on the air by the fan is only W/n2, compared with an output of W. So the net output of work is W(1-1/n2). This has to be offset by the internal energy of the air also falling by W(1-1/n2). Consequently, the air leaves the conduit at a lower temperature than it entered.
(iii) The air emerging from the fan has kinetic energy. This can be recycled by feeding it back into the converging-diverging conduit again.
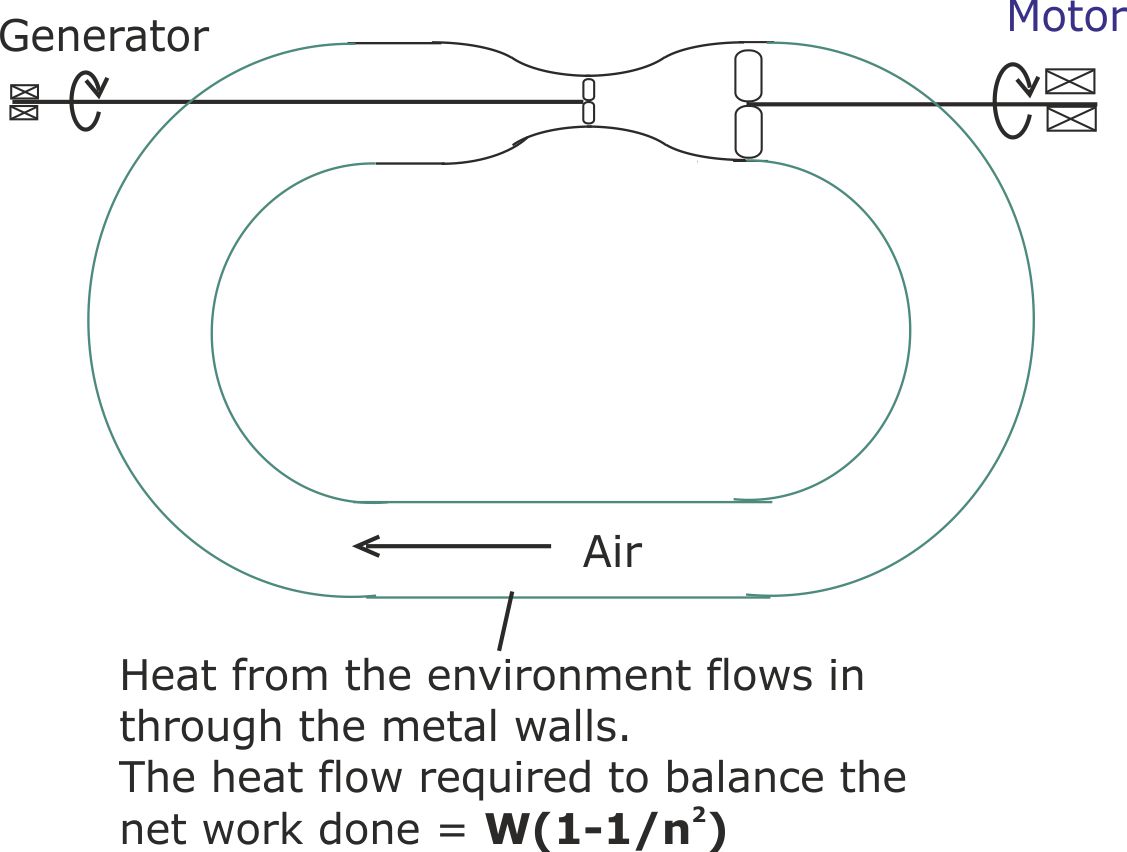
Figure 18. The completed design is counter-intuitive because it runs on thermal energy extracted from the environment. This is equivalent to being a circular heat engine that requires a cold sink.
One important difference between a convection current loop and a second generation wind turbine is that convection currents need to lose some thermal energy at the highest in the loop, in order to ensure that the top of the loop remains cooler than the bottom. In contrast, second generation wind turbine loops reduce thermal energy by doing net work.
The second generation wind turbine designs developed by Bill Courtney and Richard West are referred to as Latent Power Turbines.
These are open source technology for you to develop as you wish.
For further details, go to the Latent Power Turbines pull down menu at the top of this page.
FOOTNOTE
What if there had never been any doublethink confusion?
Power generators running on heat extracted from the atmosphere could have been invented at the dawn of the modern electrical age in the 1889s. Unfortunately the doublethink interpretation of thermodynamics created a blind spot in scientific thinking and we ended up inventing hot and dirty power generators instead.
If scientists had been more self critical during the last 130 years, doublethink would never have led us astray and we would be looking back on an entirely different history.
Here is one of these alternative histories; others are left to your imagination.
Clean power generators fuelled by atmospheric heat were invented in 1879, the same year that the light bulb was invented.
The new generators rapidly dominated the energy markets because their capital costs were highly competitive compared with fossil fuel burning engines and, apart from annual service charges; there were no running costs involved.
The productivity benefits for European manufacturing businesses were obvious, but the new generators also posed a threat because they made large scale manufacturing in the hot tropics more practical.
People doing hard physical indoor work in the tropics could now enjoy the same pleasantly cool, low humidity working conditions as northern workers, thanks to the fact that the new generators could provide cool, dehumidified air as a by-product of electricity generation.
Furthermore, tropical manufacturing offered lower production costs because raw materials such as rubber, cotton and metal ores could be sourced locally instead of being imported from overseas.
Workers in the new tropical factories required supervisors who could speak their local language. This led to a rapid expansion in the indigenous middle classes in the colonies.
By the time of the South African Boer War in 1900, British history was on the verge of repeating itself because the new middle classes in the colonial possessions wanted to enjoy the same democratic freedoms as their American predecessors had won in the American war of independence from Britain.
Britain did not have the military resources to fight the equivalent of several Boer Wars at the same time. So necessity ruled and the British colonies rapidly gained independence.
The other European colonial empires suffered a similar demise, with the parallel consequences that militarism and extreme nationalism in Europe declined too.
So, by 1914, most of the European tensions that would otherwise have led to a Great European War had been defused.
By 1918, the Europe countries had been transformed into service economies powered by very low cost electricity. The air in cities was almost pollution free and the cost of keeping warm in winter was low. Although they did not realise it, a large part of their prosperity was due to the fact that they had avoided a divisive and expensive European War.
Russia gained an extra benefit from the general European prosperity because it could charge higher prices for its grain exports. Consequently the country evolved politically, transitioning to a full democracy in 1920.
In the 1920s, there was no post-war humiliation of Germany and no reason for the rise of the Nazi Party in the 1930s. Anti-Semitism lingered on until the 1950s, but it lost its value as a political tool because the Jews could not become the scapegoats for a European war that Germany and Austria had never lost.
In the Pacific, Japan started to thrive in the early twentieth century by using clean energy instead of oil and coal. Trading made better economic sense than warfare, so it established good relationships with China and Australia to meet its raw material needs.
Consequently, militarism became redundant in Japan and there was no invasion of Manchuria and no attack on Pearl Harbour in 1941.
China had become a democratic republic in1912 and has remained as a democracy ever since.
The Second World War never materialised because by 1940, most counties were enjoying self sufficiency in terms of energy and basic foods.
There was no need for nuclear power and no incentive for countries to develop nuclear, chemical or biological weapons.
By 2000, the world had been largely peaceful for 100 years and the inhabitants of tropical countries were enjoying similar high standards of living to their old colonial masters in the north.
There had been a massive expansion of science research in the tropics, with the whole world benefiting in terms of improved healthcare and clean food production. In order to eliminate the use of pesticides, all staple foods were now grown in filtered air, either in glasshouses or vertical farms.
People continued to enjoy eating meat, fish and dairy products. But by 2023, low cost energy allowed these foods to be grown at a cellular level in vats, similar to those used for brewing beer.
Low cost clean energy also made recycling cost effective and the vast majority of waste materials were recycled.
A small fraction of the landscape was now covered in ugly food production glasshouses, but these were well hidden because most of the agricultural land had been freed up for rewilding. People had leisure access to these wild areas and humanity was finally learning to live in harmony with itself and the rest of nature.
As the world became more secure and prosperous, xenophobia became disconnected from its tribal survival origins and racial discrimination became incomprehensible to most people.
Thanks to good nutrition and healthcare, people lived long lives. So in spite of average family size falling to replacement rate in the 1960s, the world population had grown to 5 billion in the 1990s. Since then, the world population has remained stable.
Environmental pressure groups are still needed to ensure that humanity does not become too indulgent at the cost of degrading the environment. This work is powered more by hope than by anger because we are living in an environmentally improving world that we are at peace with.
********
You are unlikely to have shared in this New Eden, because with radically different economic, migration and cause of death patterns, your parents are unlikely to have met and mated. So, that’s one (good) consequence you can thank those double thinking scientists for.
